Table of Contents |
guest 2025-12-28 |
Questions and Answers
Registration
Webinar #5: Targeted Method Refinement with Skyline
|
Thanks to the people who attended Webinar #5 on March 10th, 2015, which featured a tutorial by Principal Developer Brendan MacLean on Skyline targeted method refinement. Additionally, guest speaker SRM Researcher Jim Bollinger presented on assay refinement for clinical use. Below are the supporting files for Skyline Tutorial Webinar #5 Targeted Method Refinement with Skyline including presented slides and a composite recording of the sessions which has a table of contents allowing for quick navigation through the sections of the webinar. Below is also the tutorial data sets used in Brendan's demo and there is also an associated tutorial Targeted Method Refinement which is available here. The answers to the questions posed during the Q&A portion of webinar are now posted here. NOTE: if you asked one of the questions during the webinar, be sure to check out Brendan and Jim's written responses as in a couple of them, they acknowledge that the written answer have been updated. -- The Skyline Team
|
Review Webinar 4Targeted Method Design Review Webinar 6Effective Data Processing and Interrogation Targeted Method Refinement TutorialFor those who want even more practice, the 25-page tutorial Targeted Method Refinement provides a great "hands-on" review of the concepts and processes covered in Webinar #5. |
Questions and Answers
|
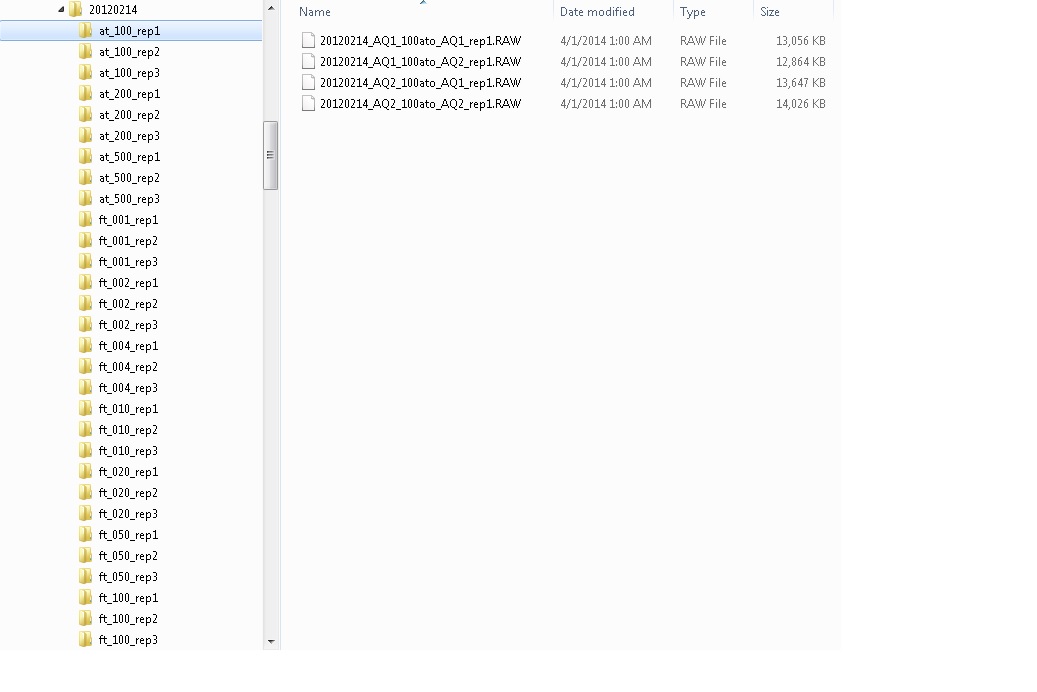
Dear Skyline Users, People were asking questions during our fifth Skyline Tutorial Webinar on targeted method refinement. Jim and Brendan have provided the answers! Q: Any comment on MRMs of deamidated peptides that could potentially share many y/b ions, be within Q1 isolation windows (for 2 and greater z) and be very similiar Rt? Ans: I misunderstood this question at the AM webinar and answered about pyro-glutamate instead of deamidation. SRM parameters typically function at unit mass resolution or better... However, if you must relax your isolation window on Q1, I would recommend monitoring for both residues (amidated and deamidated) to get a feel for the kinetics and to verify that the two peptides are resolved by chromatography -- as I expect they would be. P.S.deamidation occurs more readily when the affected residue is adjacent to glycine. Q: Can you explain how you use iRT for method refinement? Ans: I hope we will get the chance to cover iRT in a future webinar. For now you can consult the iRT tutorial: https://skyline.gs.washington.edu/labkey/wiki/home/software/Skyline/page.view?name=tutorial_irt If you have an existing iRT library, just as we had an existing spectral library in the webinar, this would allow you to measure a much larger number of peptides, scheduling them in the first round of refinement. You would first measure your standard peptides in matrix, and use those measurements to schedule your initial acquisition. That would have yielded a lot fewer runs than the 39 used in the tutorial. More details in a future webinar. Q: For DBS, what cards are used and how much blood is spotted? Ans: Standard Whatman 903 paper was used and 80-100 uL per blood spot. Each 3 mm punch taken from an individual blood spot corresponds to 3-3.5 uL of blood Q: How do you account for Hematocrit in Whole blood from different patient? Ans: Currently addressing with a larger population study with matching dbs (MS/MS) & plasma (Immunoassay) samples. Widespread application of DBS in newborn screening programs suggest this issue is often overstated. Q: Where can the heavy labeled IS protein can be purchased? Ans: Cambridge Isotope Labs offers the 15N-APOA1 that we used in this study. You can also make heavy proteins using a variant of the In Vitro Translation Kit mentioned in the presentation. Q: Once a protein is identified how long does it take to make cDNA? Ans: We can purchase cDNA constructs from the DNASU repository when a target is not already in our library for about $45.00 and clone it into one of the vectors compatible with the IVT kit. Q: If you removed a bad raw file from the skyline analysis and deleted this bad raw file from the folder where you import your data, then imported a new raw file with the same name as the bad file, how can you make skyline "forget" the bad file and import/read the new good file? Ans: The key here is to know that Skyline does not commit changes to its .skyd data file until you save. This is to support simple Undo, without needing to reimport data. But, this does appear to be confusing to people. Perhaps in a future release, I will force a save before showing the File > Import > Results form. Although, that will make impossible the trick I showed during the webinar of re-organizing your imported data without re-importing it. For now, just remember to save after you make any change to imported results that you want to commit to the files on disk. Q: What is the effective way to lyse red blood cells either in whole blood or DBS? Ans: The RBC in DBS samples should already lyse during the drying step. There are a variety of options for lysing rbc’s in whole blood from osmotic stress to proprietary buffers. Q: Do you take ratio of the peptide to quantify the protein and how transitions per peptides are used? Ans: Q: Have you tried Agilent cards? How do they compare to Whatman? Ans: I have not tried the Agilent cards but would gladly welcome a few free samples. ;) Q: How the peptide quantification can be converted into the protein quantification and how many peptides need to be selected for this purpose? Ans: Q1: calibration curves with analytical standards. Q2: The more the merrier but I usually try to shoot for at least 3 peptides per gene product when possible. Q: I didn't catch how to handle the calibration curves in multi directory files. Can you have all the replicates and all concentrations points in a same view? Ans: Now that I read this, it is unclear whether the question was simply about how to handle the instrument vendor formats that use directories (Waters .raw, Bruker and Agilent .d) instead of files (Sciex .wiff, Shimadzu .lcd, Thermo .raw). If that was the question, then the answer is simply that the Skyline Import Results form makes all data files and directories appear similar, and you can import the directories just as if they were files. During the webinar, however, I answered this question in the context of what I had been talking about in the webinar, which was how to handle complex multi-replicate data sets that require multiple instrument injections to cover all of the peptides in a document, and I gave the example of a calibration curve experiment I had been working on that required 4 injections to cover all peptides for 10 concentrations, measured in technical triplicate to produce 120 raw data files. This would be pretty painful to import the 30 separate Skyline “replicates” with 4 files each using the “Add one new replicate” option first used in the tutorial. Instead I suggested organizing the raw data files into a directory structure using the Windows explorer (as I actually did) and then importing the data using the “Add multi-injection replicates in directories” option. The directory structure I used, looked like this: |
Registration
|
Dear Skyline Users, The Skyline Team would like to thank over 200 people that attended our fourth Skyline Tutorial Webinar titled Targeted Method Design with Skyline in February. For anyone who would like to review what was presented, check out the resources available - including taped video of the full webinar, presentation slides and supporting data files. We are very excited to announce our next webinar in the series: Webinar #5: Targeted Method Refinement with Skyline [registration closed]
This webinar will include an introduction and tutorial from Brendan MacLean, Skyline Principal Developer, and a presentation from Jim Bollinger on method refinement for his "Wellness Assay". Join us, learn and help us to better meet your targeted proteomics research needs. --Skyline Team |
Presenters
Brendan MacLean 
Jim Bollinger |
Review Webinar 4Targeted Method Design with Skyline Week-Long Course at
|